Research into lithium-oxygen batteries could boost performance of electronics, cars
Tue, 01/29/2019
LAWRENCE — Sick of having to plug in your phone every night? Help might be on the way.
New research at the University of Kansas could provide longer-lasting batteries for most consumer electronics and electronic vehicles in the coming years.
Today, most Americans own electronic devices powered by rechargeable lithium-ion batteries and some drive cars powered by lithium-ion battery technology. But lithium-ion batteries have drawbacks, like the need for regular recharging.

“Everyone wants to have better batteries for phones, electronics and cars,” said Xianglin Li, assistant professor of mechanical engineering. “The current lithium-ion battery, which is used everywhere, doesn’t have enough energy density — you have to charge your phone every day.”
Recently, Li earned a new $219,312, two-year grant from the National Science Foundation2 to push forward cutting-edge lithium-oxygen batteries. He said that lithium-oxygen batteries represent the most promising battery platform to take the place of lithium-ion.
“The research we’re doing on the lithium-oxygen battery represents the next generation of energy storage,” he said. “Theoretically, it has about one order of magnitude higher storage capacity than lithium-ion. So, if you switch to this in the future, you’ll only need to charge your phone once a week. There are competing technologies like the zinc-air or lithium-sulfur batteries, but lithium-oxygen clearly is the one with the highest capacity, so it has great advantage.”
While lithium-oxygen batteries promise much greater energy storage capacity, their shortcoming is an inability to discharge energy as fast as lithium-ion batteries. Until this drawback is overcome, lithium-oxygen battery technology will remain in the lab research stage, according to the KU investigator.

“The problem is lithium-oxygen has low current density — it lasts a long time, but you don’t get a lot of power,” Li said. “If you use lithium-oxygen batteries for an electric car, you could drive 500 miles, but you can’t accelerate very fast. Driving just a few miles per hour isn’t very fun. As far as I know, almost all lithium-oxygen batteries are still in the research phase and the technology doesn’t have a very large market yet. Performance, stability and lifetime are all issues for lithium-oxygen batteries now. But in the ’70s and ’80s, lithium-ion batteries had similar issues.”
With his new NSF grant, Li hopes to develop technology to boost the current density of lithium-oxygen batteries to make them more practical. He’ll work in the X-ray Computed Tomography Facility at Carnegie Mellon University, collaborating with Shawn Lister.
“Our objective is to increase the power of lithium-oxygen battery by one order of magnitude while having the state-of-the-art energy density,” Li said.
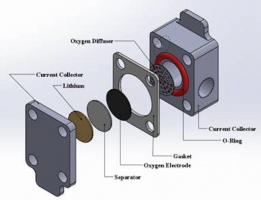
Li and Lister will focus on understanding and improving the function of the oxygen electrode of the lithium-oxygen battery. Li said lithium-oxygen batteries must absorb oxygen from the air through nanoscale pores to facilitate reactions. So, the electrochemical performance of lithium-oxygen batteries depends on the liquid-gas two-phase flow at the pore scale of the electrode. The researchers aim to better understand the pore-scale transport of the battery electrodes as governed by pore size, structure, connectivity and wettability.

“Shawn Lister at Carnegie Mellon has a unique device to measure morphologies at the nanoscale — technology that’s like a CT scan in a hospital, but with very high resolution down to the 20-30 nanometer resolution,” Li said. “We want to measure the lithium-oxygen battery electrodes and understand how we can transfer oxygen better with an improved design. The battery has to absorb oxygen from the air, so if we don’t supply oxygen fast enough, the power will be limited. We’re going to use his facility along with our advanced models and theories to try to design a high-performing battery electrode — and hopefully we’ll have a prototype for lab demonstration.”
The investigation will focus on improving oxygen’s sluggish mass transfer in battery electrodes.
“Batteries are electrochemical devices where you want a high reaction rate — and the only place the reaction can happen is in the electrode and electrolyte interface,” Li said. “We have to create as high a surface area as possible using nanomaterials, but mass transfer will be very slow because nanopores have higher resistance. In a lithium-oxygen battery, the electrolyte is liquid and the mass transfer through liquid is very slow compared with air. One example is you can’t breathe through a piece of paper soaked with water because of the high water resistance to oxygen transfer. It’s the same case for a liquid electrolyte, so we want to create the gas phase in our electrode to facilitate the oxygen transfer.”
Li said the project has the potential to result in a patented technology that could push forward research and adoption of the lithium-oxygen technology in the coming years. The researchers plan to form potential partnerships with the local industry and reach out to the public through the Kansas City STEM Alliance3.
Additionally, the grant work will support the training of two KU graduate students.
“I currently have a Ph.D. student who I think will join with me in the summer to come to CMU,” Li said. “It’s a great training experience. He’ll graduate later this year and next year there will be another graduate student — so I’ll train two different students during this project.”
Top image: Battery design will focus on the oxygen electrode (~1.2 cm diameter and 0.4 mm thickness). Credit: Xianglin Li
Top right image: Researchers will obtain 3-D nanotomography of the electrode. The 3-D nanostructure will enable further simulations of mass transfer coupled with electrochemical reactions and improvement of electrode designs. This image represents simulated oxygen distribution within the electrode. Red means higher concentration, and black means lower concentration. Credit: Xianglin Li
Bottom right image: Nanotomography of battery electrode collected from Argonne National Lab. Different colors represent different materials. Credit: Xianglin Li.

